Health
Results: 385-396 of 7366

Compass Pathways Announces Third Quarter 2025 Financial Results and Business Highlights Including Acceleration of Commercial Launch Plans by 9-12 Months

Invitation to presentation of BioArctic's third quarter report for July - September 2025 on November 13 at 9.30 a.m. CET
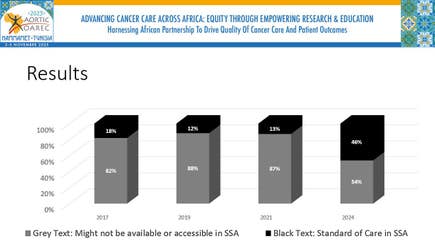
NCCN Celebrates Expanding Access to Cancer Treatment in Africa at 2025 AORTIC Meeting with New NCCN Adaptations for Sub-Saharan Africa

Therme Group Awarded Tender by Singapore to Build Asia's First State-of-the-Art Wellbeing Destination